Consumers often participate in a variety of studies, including medical and other studies that require them to follow a specified regimen and record their reactions, physically and mentally meeting the regimen’s requirements. Before participating, they are requested to read and sign an informed consent form. Perhaps you have read about studies where half the group is taking a placebo while the other half is taking the medication that is being studied. Neither group is aware of whether they are taking a placebo or the real thing.
This post discusses the informed consent form, why it is required, and the essential elements that should be in every informed consent. Participants in these studies should always be aware of the study’s objectives and the potential impacts on their person.
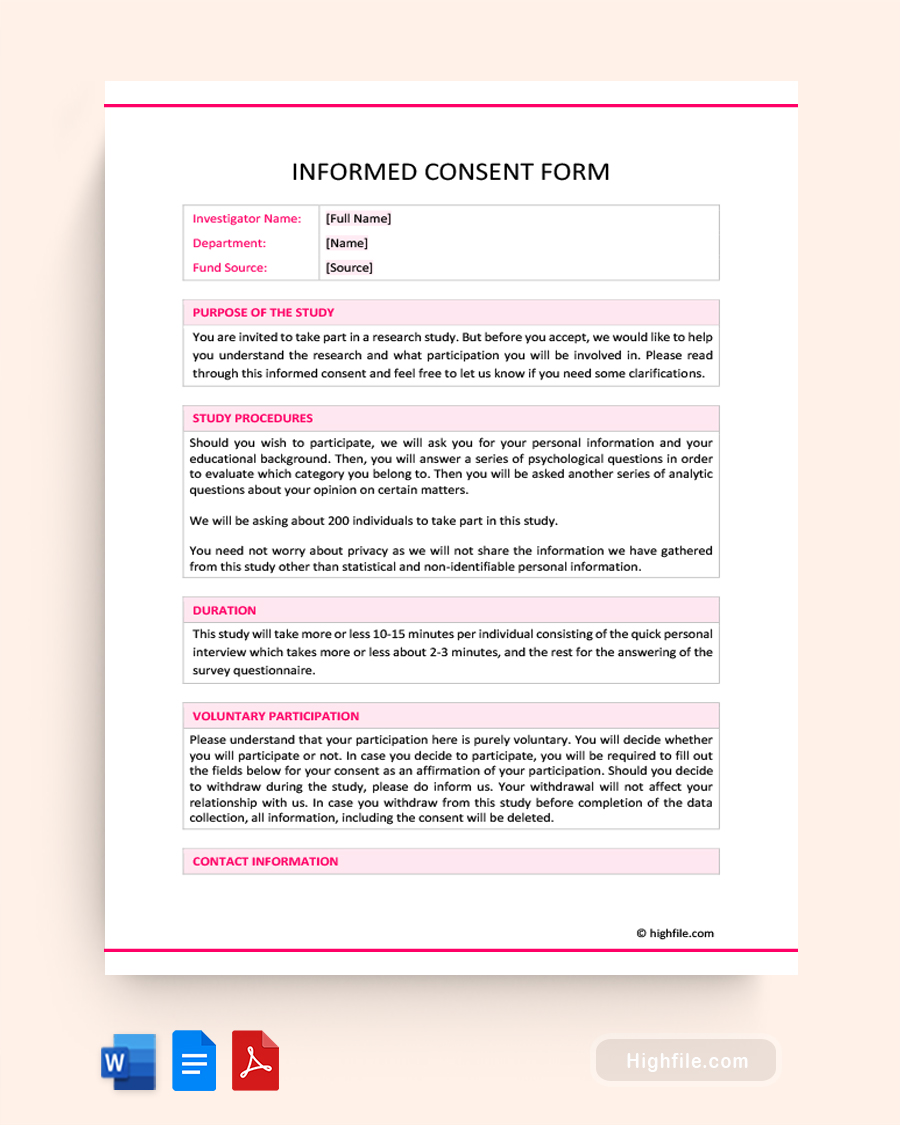
What Is an Informed Consent Form?
An informed consent form is a document that informs research/study participants about the study they are about to participate in and what is expected of them during the study. It also should inform them of any risks associated with the study. Most studies and research projects require that the participant sign the informed consent form to signify they have read the form, agree with participation in the study, and are aware of any risks associated with participation.
Research projects involving the collection of bio-specimens for genetic analysis require documented signed consent.
It is also considered best practice and ethically required to request a signed informed consent form for any research projects that are considered exempt by the IRB. Subjects that are part of vulnerable populations, including children, prisoners, or cognitively impaired individuals, must be willing to participate willingly and be informed of the study/research they will participate in.
Children must provide their consent as well as their parents before including them in any research studies.
The Informed Consent Process
The informed consent form process involves the investigator leading a research project or study of some kind and the participant or interviewee. The investigator should discuss the project and the contents of the consent form. The interviewee should read the consent form thoroughly and ask any clarifying questions they may have. Once they have read the form and have had all of their questions addressed, both the investigator and the participant should sign the form. Both persons should also print their names and add the date of the signature.
In situations when a child is a participant, a parent or guardian should be present, and consent should be obtained from both the child and the parent or guardian. If the participant cannot read the form or does not understand the form, a guardian should read the form and explain the investigation to the participant. Both parties should sign the form in addition to the investigator.
All parties signing the form, including the investigator, the participant, and/or the guardian, should be given copies of the signed consent form.
Essential Elements of an Informed Consent Form
There are typically five major elements of the informed consent form.
- Statement – indicating that the project is a research or study sponsored by the name of the department and all participation in the project is voluntary.
- Summary – of the research or study project and should include the purpose, how long it will last, and a summary of the procedures or expectations
- Risks – should be identified associated with the research or study, if any, and also any discomforts that may be experienced by the participants
- Benefits – expected from the results of the study that are reasonable and specific to the participant and the project or research field
- Procedures – in the case of a research project, there may be alternative procedures or different treatments that may be available to the participant. These should be identified and explained to the participant.
- In addition to the five major elements, investigators preparing informed consent documents should keep in mind the following:
- Write in plain language at levels the subject population should be able to understand
- Write at an 8th grade reading level
- Avoid technical jargon and complex project-related terminology
- Ask a friend or colleague to read the document to assess comprehension
FAQs
The following are several frequently asked questions many investigators and participants may have.
Informed consent is needed when the collection of bio-specimens is taken for genetic analysis. Research that is considered exempt by the IRB-HSBS should also consider using an informed consent document to comply with ethical best practices.
In today’s electronic world, asking for a participant’s informed consent can be accomplished in several ways. These include:
ᐅ Signed/written consent – form signed and dated before participation
ᐅ Online consent – before the start of the research, use an opening statement before beginning an online survey
ᐅ Oral (recorded) consent – an explicit statement that is recorded asking for and receiving the participant’s consent before beginning the interview
ᐅ Passive or tacit consent – is assumed if the participant does not object to their participation after being informed about the study or project.
Whether you are following a written, online, oral or passive consent approach, the participant must meet these criteria:
ᐅ Must have the mental capacity to consent
ᐅ Consent must be voluntary
ᐅ The patient must be fully informed regarding the project and what they are consenting to.
Informed consent must be voluntary, and the participant should have the complete freedom to make their own decision regarding providing consent and participating in the study. The following factors can invalidate informed consent:
ᐅ Involuntary consent
ᐅ Fear of injury
ᐅ Intimidation
ᐅ Misunderstanding
ᐅ Misrepresentation of the facts
ᐅ Misconception of the facts
Ultimately it is up to the courts to determine if informed consent and the participant’s agreement are legally binding. However, the intent appears that a properly written consent form, signed by both parties, is legally binding. If you have any doubt about the form, the project, etc., ask your lawyer to review the consent form and explain the legal obligations of the form.
Unless a time limit is specified on the original consent form, informed consent is considered valid for a specified procedure until the participant revokes the consent or after three years. After three years, the original informed consent form is to be destroyed.
The participant or patient signs the informed consent form except for children or those consumers who are unable to for a variety of reasons, including disabilities and inability to understand the contents of the form.
Guardians of children and disabled participants can sign on behalf of the participant; however, guardians are required to explain all of the details of the project or procedure to the participant.
Signatures are usually required for informed consent situations. Both the participant and the investigator must sign the consent form and date the signatures. The participant’s legally authorized representative, i.e., the children’s guardian, must sign the consent form on their behalf.
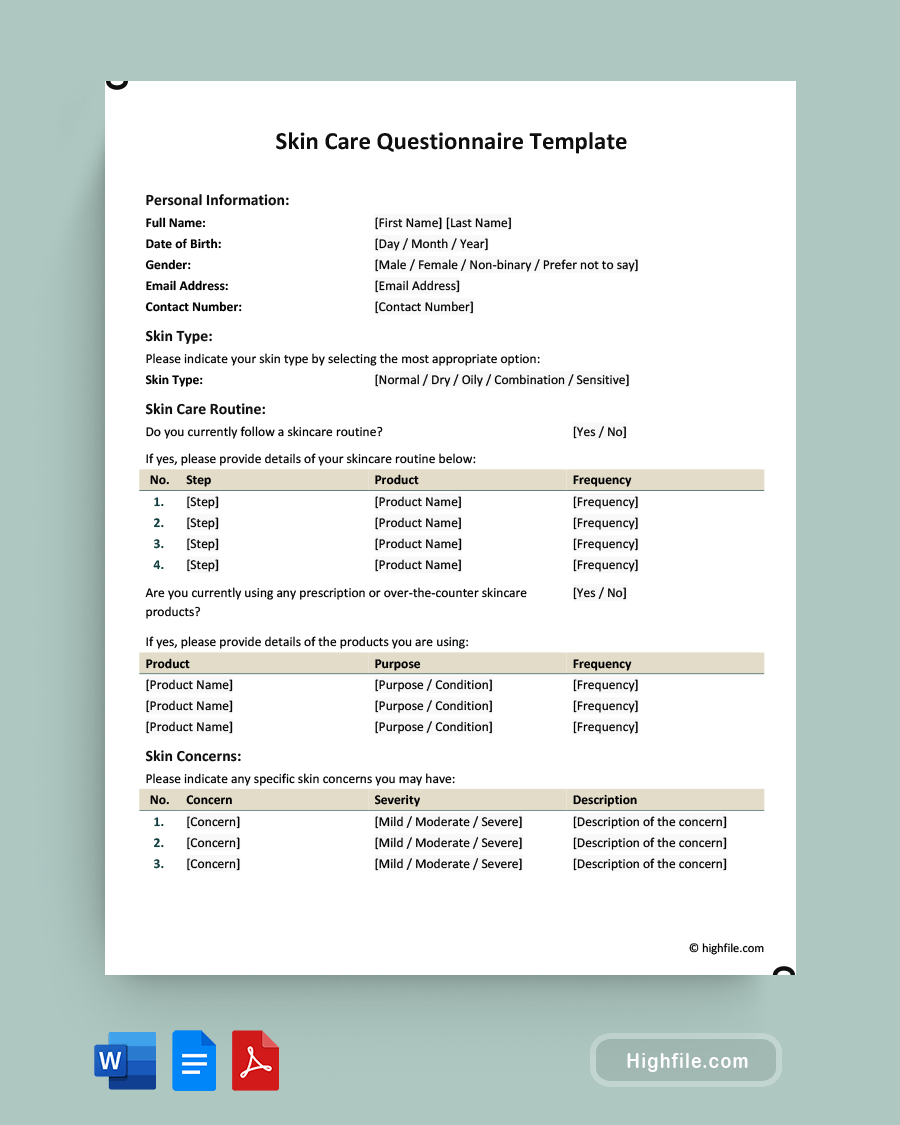
The following information should be included on the informed consent form.
A statement describing the research or study sponsored by the name of the department and all participation in the project is voluntary. The summary of the research or study project should include the objective or purpose, how long participation will last, and a summary of the procedures. The risks to the participant should be identified as well as also any discomforts that may be experienced by the participants. The benefits the participants or society should expect from the results of the study are reasonable and specific to the participant and the project or research field. The procedures that will be followed and any alternative procedures or different treatments that may be available to the participant.
Key Points
A proper informed consent form should be tailored to the project, survey, or research that is to involve participants and require them to take part in a procedure of some sort. The document should cover what is expected of them, the duration of their participation, and a description of any procedures they will be involved in.
Both the investigator and the participant should sign and date the consent form. If the participant is a child or unable to sign the consent form due to a disability, their guardian should sign on their behalf after explaining to the participant what will be expected of them.
Copies are to be provided to all parties, including the participant, the investigator, and the guardians.
Where possible, the consent form should specify the duration of the consent or if it is limited to a specific procedure. Informed consent is considered legally binding; however, a final decision is ultimately up to the court. Consent forms can be invalidated if they were not voluntarily provided if there is fear of injury, intimidation, a misunderstanding, or misrepresentation of the facts or misconception of the facts.