Taking care of your health is vital, which means seeing a doctor, but before anyone receives treatment, they have to consent. The same is true when performing a case study or other research. Patient consent forms are an essential part of any medical practice. They provide a legal document outlining the rights and responsibilities of the patient and show that they agreed to the treatment. It is essential for all parties involved to understand what is included in a patient consent form so that patients can make informed decisions about their healthcare. Hence, we recommend using a high-quality professional patient consent form template to lay out the framework for your documents.
This article will discuss the essential elements of a patient consent form, including a list of items that should be included in every form, why you need them, and best practices for getting patients to sign them.
What Is a Patient Consent Form?
A patient consent form is a document that medical professionals use to ensure that their patients know the risks and benefits associated with any medical procedure, case study, or research they are about to participate in. It also serves as a legal agreement between the doctor and the patient to show that both parties understand the terms of the procedure. Informed consent forms are a vital part of any medical practice, as they help protect both doctors and patients from potential legal issues. By signing a consent form, patients can be sure that their rights are respected and that their doctor has taken all necessary steps to inform them of the risks and benefits and protect their safety.
Why is Patient Consent Form Important?
Patient consent forms are an important and legally required part of the healthcare process. They provide a way for doctors and other healthcare providers to ensure that patients understand their rights and the risks associated with any medical procedure. Additionally, they prevent the potential legal liability of not obtaining informed consent.
By having a patient sign a consent form, healthcare providers can build trust with their patients by ensuring that they are fully informed about the risks and benefits of any medical procedure. It gives patients an opportunity to ask questions and get answers from their doctor before making any decisions. Below is a list of why you need a patient consent form.
- Informed Consent- Doctors and other medical professionals are (almost always) required to obtain informed consent before treating their patients.
- Licensing- Unless the patient is incapable of consent because they are dying or incapacitated, medical professionals can lose their licenses for treating patients without consent.
- Liability- Treating a patient without their consent is considered malpractice and could land you in court, facing fines or worse. You might even risk a public rebuke or loss of license.
- Communication- In addition to providing vital information to the reader, a patient consent form creates an inroad for dialogue so you can answer their questions. This builds trust and helps satisfy the ‘informed’ part of consent.
- Treatments and Alternatives- In addition to answering any patient questions, you must also discuss the proposed treatment and any possible alternatives. A well-written consent form shows that this requirement has been met.
- Documentation- A paperwork trail is part of every medical transaction, from a simple checkup to brain surgery. Documenting the process is part of your duty. It helps other doctors and medical professionals who see that patient in the future to understand their medical history.
Tips on Getting Patients to Sign a Consent From
The process of getting patients to sign a medical consent form can be daunting for both the patient and the doctor. Miscommunication is easy, and so is forgetting that your patients don’t have the benefit of your years of education to help them understand complex industry-specific terminology or why something is necessary, especially when it sounds painful. Doctors must provide clear and concise information to their patients so that they can make informed decisions about their health care. To ensure that this process goes smoothly, here are some tips on best practices for getting patients to sign a medical consent form.
- Questions and Answers- It is essential to answer all patient questions honestly and thoroughly. Help them understand what they are signing up for and ensure they know the benefits, risks, and alternatives, so they are comfortable with the procedure or treatment.
- Patience and Tone- Remember your bedside manner when dealing with curious, frightened, or difficult patients. This is a daily job for you, but it’s a relatively rare experience for them.
- Terms and Definitions- Use smaller non-medical words when explaining complex terms. This will help patients better understand what they agree to without feeling overwhelmed by medical jargon. If they don’t get it, explain it in a different way.
- Treatment Recommendations- Explain procedures, risks, benefits, and side effects simply and in plain language so that everyone involved understands what is being discussed and what the process entails.
- Benefits- From better health to remuneration or societal benefits, make sure the patient understands why it is important for them to consent.
Essential Elements of a Patient Consent Form: Case Study or Publication
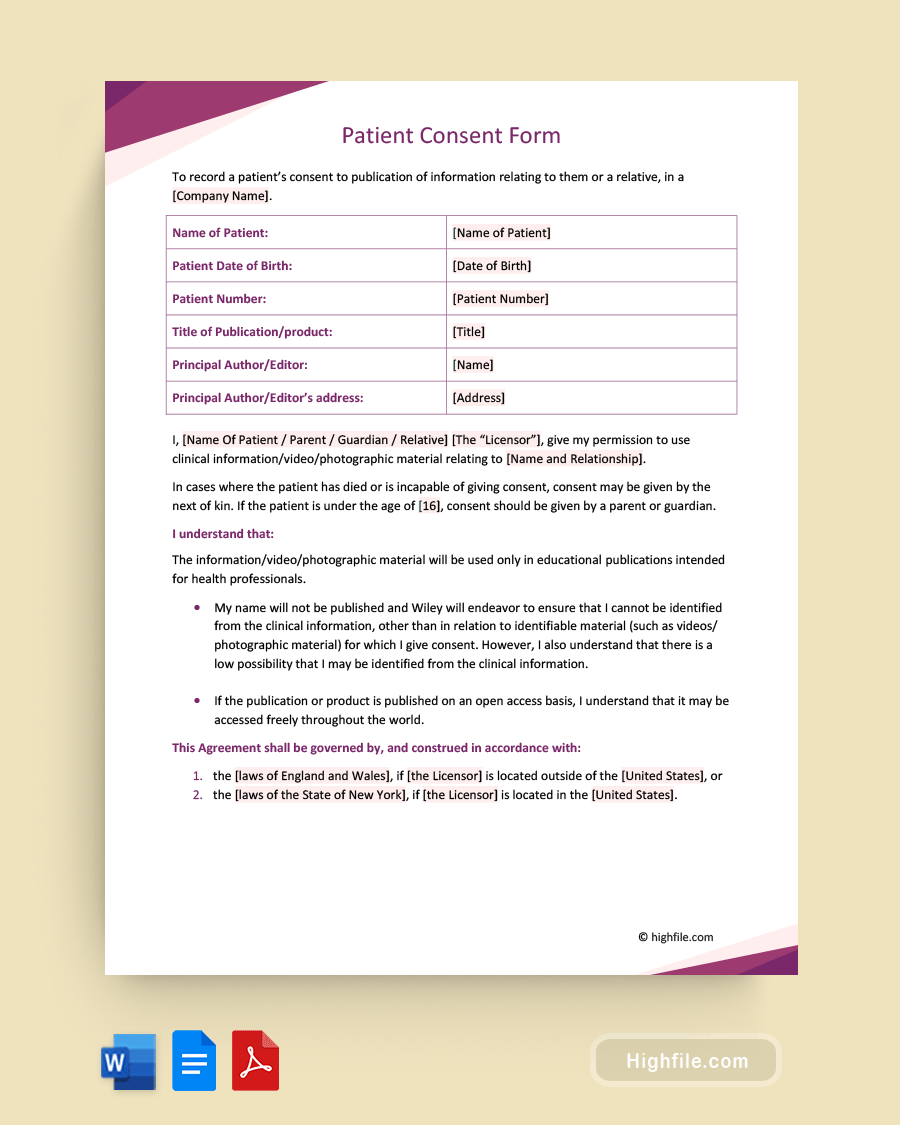
The essential elements of a patient consent form should indicate what the document is for, such as a case study or research study, and who is conducting it or doing the writing. Additionally, it should clearly indicate what the patient is agreeing to and that they understand the terms of the agreement and its intended use. Below is an outline of the critical aspects of this type of document in descending order of where it belongs on the page.
- Form Owner- Your logo, clinic or practice name, study name, or other identifying formal title belongs at the top of the page.
- Document Title- It should say “Patient Consent Form” or something similar to show what this document is in large, bold print.
- Name- Put the patient’s name at the top of the page.
- DOB- The date of birth of the patient.
- Patient Number- Patient registration number.
- Product Title or Name: The name of the case study, paper, or product
- Author Name and Contact Information- The name of the principal author or editor and their (business) address or other contact information
- Consent Statement- The consent statement usually says something to the effect of, “I (patient name), give my permission to (author or company) to use (specific named medical records) for (purpose) including publication.
- (Optional if Relevant) Remuneration Statement- This shows what, if anything, the patient will receive as compensation
- Acknowledgment of Purpose and Intended Use Statement- This statement says that the patient knows why you are asking for the data, whether they will be named, and who can access the information once published.
- Governance Statement- A governance statement shows which laws govern the publication.
- Signature and Date Lines- Have the patient sign and date the form like any other legal document, with a handwritten signature unless it is an e-form.
Important Note: If the patient has passed away, their next of kin can give written permission to release their records, images, videos, or other relevant data.
FAQs
It can seem daunting when you’re new to creating patient consent forms, which is another good reason to use a professionally designed document template. We’ve provided the answers to the most frequently asked questions people had after inquiring about patient consent forms. Here you’ll find answers about why patient consent matters and more to help you and your patients with consent forms.
You need patient consent because they have the right to determine what will happen to their bodies. Informed consent is an essential part of any medical procedure or clinical trial that shows you have the right to give treatment. It is the proof you’ve obtained a patient’s voluntary agreement to receive medical care or participate in a research study. A patient consent form shows that the patient understands the risks, benefits, and alternatives associated with the proposed treatment or research study before agreeing to proceed. It also helps protect the patient and healthcare provider from potential legal issues that could arise from a lack of informed consent.
You need patient consent for a case report because informed consent is mandatory. Without patient consent, the publication of a case report can be rejected and may even damage the patient-doctor relationship or the author’s professional credibility. It is also important to note that informed consent should not be taken lightly as it can have profound implications if not appropriately obtained. In fact, informed consent is required for any medical research involving human subjects, including publishing a case report. This helps ensure that all ethical considerations are taken into account when obtaining informed consent from patients before publishing their case reports.
When a patient does not give consent for a medical procedure or case study, you cannot treat them or publish information about them. Doing so is unethical and could lead to severe consequences. If caught practicing medicine on patients or publishing without informed consent, a medical professional could face public reprimand, job loss, and loss of license. Typically you’ll receive a warning or letter of concern first. However, in some cases, the doctor could be sued or charged with battery if they perform a procedure without consent. It is essential to take all necessary steps to ensure that you have obtained valid consent prior to treating or writing about every patient.
Final Thoughts
Patient consent forms are essential to the healthcare process, as they ensure patients understand their rights and responsibilities regarding their health. Moreover, signing these crucial documents shows they have been provided the necessary information to give informed consent. Case studies also require informed consent, even if the patient isn’t named or receives only a placebo. Since informed consent is a legal requirement for any case study or treatment, healthcare providers must understand the best practices for obtaining patient consent. Using a preformatted, professional patient consent form template creates the framework for your documents. It helps ensure you don’t miss any vital information, and it helps save time so you can do more important work.